Sunday, October 3, 2010
Friday, October 1, 2010
Thursday, September 16, 2010
Monday, September 13, 2010
REDOX - electrolysis as aqueous of molten?
Only copper ions, silver ions and gold ions can undergo electrolysis as an aqueous solution.
Every other metal ion needs to be in its molten state.
Why?
Because in an aqueous solution there is also H+ and OH- ions present H2O --> H+ + OH-
and so the H+ ions compete for the cathode with the metals ions.
Because all metals except Cu, Ag, Au are more reactive than H, the H+ ions will be reduced to H2 before the others.
(remember the more reactive the metal is the more stable the metal ions is, and so the harder it is to reduce.)

Every other metal ion needs to be in its molten state.
Why?
Because in an aqueous solution there is also H+ and OH- ions present H2O --> H+ + OH-
and so the H+ ions compete for the cathode with the metals ions.
Because all metals except Cu, Ag, Au are more reactive than H, the H+ ions will be reduced to H2 before the others.
(remember the more reactive the metal is the more stable the metal ions is, and so the harder it is to reduce.)

REDOX - electrolysis
REDOX -terms
When answersing questions on REDOX, you must always think about what is happening to the electrons, what chemical is gaining and what chemical is losing electrons.
Oxidised = has lost electrons (LEO)
Reduced = has gained electrons (GER)
Oxidising agent/ oxidant = the chemical that is itself reduced, so it has gained electrons.
Reducing agent/ reductant = the chemical that is itself oxidised, so it has lost electrons.
Oxidised = has lost electrons (LEO)
Reduced = has gained electrons (GER)
Oxidising agent/ oxidant = the chemical that is itself reduced, so it has gained electrons.
Reducing agent/ reductant = the chemical that is itself oxidised, so it has lost electrons.
REDOX -Halogens as reducing agents
Colours of the halogens:
All halogen ions (Cl-, Br-, I-) are colourless.
Fluorine (F2) - Pale yellow
Chlorine (Cl2) - Green
Bromine (Br2) - Orange
Iodine (I2) - Brown
F2 is the strongest reducing agent because it is the most reactive and 'wants' to be F- ions more than the other halogens.
F2 is able to displace any other halide ion from solution.
example:
F2 + 2NaBr --> 2NaF + Br2
(F atoms) (Br- ions) (F- ions) (Br atoms)
Pale yellow Orange

All halogen ions (Cl-, Br-, I-) are colourless.
Fluorine (F2) - Pale yellow
Chlorine (Cl2) - Green
Bromine (Br2) - Orange
Iodine (I2) - Brown
F2 is the strongest reducing agent because it is the most reactive and 'wants' to be F- ions more than the other halogens.
F2 is able to displace any other halide ion from solution.
example:
F2 + 2NaBr --> 2NaF + Br2
(F atoms) (Br- ions) (F- ions) (Br atoms)
Pale yellow Orange

REDOX - Oxidation numbers
NOTE:
There are some things you MUST put into your answers to questions about Oxidation numbers.
1. If the oxidation number goes up - the chemical has been oxidised and so has lost electrons.
2. If the oxidation number goes down - then the chemical has been reduced and so has gained elections.
Assigning Oxidation numbers

There are some things you MUST put into your answers to questions about Oxidation numbers.
1. If the oxidation number goes up - the chemical has been oxidised and so has lost electrons.
2. If the oxidation number goes down - then the chemical has been reduced and so has gained elections.
Assigning Oxidation numbers

Monday, September 6, 2010
Sunday, August 29, 2010
Monday, June 28, 2010
The titration method
The titration sequence of events:
1. Rinse a conical flask with water.
2. Rinse the pipette with the HCl solution and then pipette, a 25 mL sample of the hydrochloric acid and place it in a conical flask, (an aliquot). Repeat so that you have 3 conical flasks set up and ready to go.
A pipette is a piece of apparatus which accurately delivers a given volume -before filling it is always rinsed with a small amount of the solution.
3. A few drops of an appropriate acid-base indicator, in this case phenolphthalein, is added to the flask. The solution will remain colourless.
4. A burette is then rinsed with a sample of sodium hydroxide (the solution it is to be filled with) and then filled to just below the 0.00 mL mark
A burette is another piece of glassware that accurately measures out a volume of liquid.
5. The initial volume of aqueous NaOH in the burette is carefully read (to 2 decimal places - giving an accuracy of ± 0.02 mL).
6. Carefully add the NaOH to the aqueous HCl, finishing the titration as soon as the first permanent pink colour is observed.
The colour change is referred to as the end-point, and assuming the correct indicator has been used, is the point when the acid and base have reacted in the molar ratio given by the balanced neutralisation equation.
7. By taking the difference between the initial and final volumes in the burette, the total volume of NaOH(aq) added can be carefully determined.
8. The titration is repeated at least 3 times, or until you have obtained 3 concordant results i.e. burette volumes (titres) that, ideally, agree to within ± 0.2 mL.
1. Rinse a conical flask with water.
2. Rinse the pipette with the HCl solution and then pipette, a 25 mL sample of the hydrochloric acid and place it in a conical flask, (an aliquot). Repeat so that you have 3 conical flasks set up and ready to go.
A pipette is a piece of apparatus which accurately delivers a given volume -before filling it is always rinsed with a small amount of the solution.
3. A few drops of an appropriate acid-base indicator, in this case phenolphthalein, is added to the flask. The solution will remain colourless.
4. A burette is then rinsed with a sample of sodium hydroxide (the solution it is to be filled with) and then filled to just below the 0.00 mL mark
A burette is another piece of glassware that accurately measures out a volume of liquid.
5. The initial volume of aqueous NaOH in the burette is carefully read (to 2 decimal places - giving an accuracy of ± 0.02 mL).
6. Carefully add the NaOH to the aqueous HCl, finishing the titration as soon as the first permanent pink colour is observed.
The colour change is referred to as the end-point, and assuming the correct indicator has been used, is the point when the acid and base have reacted in the molar ratio given by the balanced neutralisation equation.
7. By taking the difference between the initial and final volumes in the burette, the total volume of NaOH(aq) added can be carefully determined.
8. The titration is repeated at least 3 times, or until you have obtained 3 concordant results i.e. burette volumes (titres) that, ideally, agree to within ± 0.2 mL.
Calculations
Rules and regulations:
You must record your calculations to as many sif figs as are on the calculator.
These sig figs must then be round down to 3 sig fig for your final answer.
You must record your calculations to as many sif figs as are on the calculator.
These sig figs must then be round down to 3 sig fig for your final answer.
Titrations
What is a titration?
In an acid-base titration the neutralisation reaction between an acid and base is used to determine the concentration of one of the reactants, if the concentration of the other is accurately known.
Using the reaction between a solution of hydrochloric acid (unknown concentration) and sodium hydroxide (standard solution - concentration known) is an example.

For achievement with excellence:
• at least three titre values must fall within a range of 0.2 mL; the average titre value must be within 0.2 mL of the expected outcome
• a titration calculation where the stoichiometry is not one-to-one must be carried out correctly using only concordant titre values. The final answer must have correct units and an appropriate number of significant figures.
In an acid-base titration the neutralisation reaction between an acid and base is used to determine the concentration of one of the reactants, if the concentration of the other is accurately known.
Using the reaction between a solution of hydrochloric acid (unknown concentration) and sodium hydroxide (standard solution - concentration known) is an example.

For achievement with excellence:
• at least three titre values must fall within a range of 0.2 mL; the average titre value must be within 0.2 mL of the expected outcome
• a titration calculation where the stoichiometry is not one-to-one must be carried out correctly using only concordant titre values. The final answer must have correct units and an appropriate number of significant figures.
Tuesday, June 15, 2010
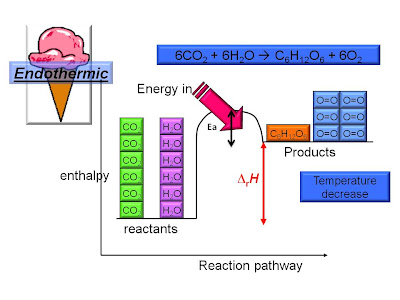
Measuring Reaction Enthalpy
Equilibrium
Kc Equilibrium constant:
eg for: N2 + 3H2 --> 2NH3
Kc = [product]/[reactant]
Kc = [NH3]2/[N2][H2]3
This is a ratio of product concentration to reactant concentration at equilibrium.
If Kc>1 then the equuilibrium position favours the product side
If Kc<1 then the equilibrium position favours the reactant side.
* Solids do NOT get included into the Kc expression
Le Chateliers Principle:
States that:
When a system is at equilibrium, that system will shift to minimise any changes made to it.
[When answering questions on equilibrium, you need to name the chemical(s) affected by the change]
Things that can change the position of equilibrium:
1. Temperature:
When heat is added, the equilibrium will shift in the endothermic direction.
When cooled the equilibrium will shift in the exothermic direction.
Kc will change as it is temperature dependant.
2. Concentration:
When adding or removing chemicals from an equilibrium you are changing the concentration. The equilibrium will shift in the direction to either replace any chemicals removed or use up any chemical added.
Kc remains the same.
3. Pressure:
When the pressure is increased (by decreasing the volume of the container) the equilibrium will shift to the side with the least number of moles of gas.
When the pressure is decreased (by increasing the volume of the container) the equilibrium will shift to the side with the most moles of gas.
Kc remains the same.
4. Catalyst:
A catalyst will not affect equilibrium position. It will just increase the rate at which equilibrium is reacted and it will increase the rate of the forward and reverse reactions equally.
A catalyst is not used up in the reaction.
eg for: N2 + 3H2 --> 2NH3
Kc = [product]/[reactant]
Kc = [NH3]2/[N2][H2]3
This is a ratio of product concentration to reactant concentration at equilibrium.
If Kc>1 then the equuilibrium position favours the product side
If Kc<1 then the equilibrium position favours the reactant side.
* Solids do NOT get included into the Kc expression
Le Chateliers Principle:
States that:
When a system is at equilibrium, that system will shift to minimise any changes made to it.
[When answering questions on equilibrium, you need to name the chemical(s) affected by the change]
Things that can change the position of equilibrium:
1. Temperature:
When heat is added, the equilibrium will shift in the endothermic direction.
When cooled the equilibrium will shift in the exothermic direction.
Kc will change as it is temperature dependant.
2. Concentration:
When adding or removing chemicals from an equilibrium you are changing the concentration. The equilibrium will shift in the direction to either replace any chemicals removed or use up any chemical added.
Kc remains the same.
3. Pressure:
When the pressure is increased (by decreasing the volume of the container) the equilibrium will shift to the side with the least number of moles of gas.
When the pressure is decreased (by increasing the volume of the container) the equilibrium will shift to the side with the most moles of gas.
Kc remains the same.
4. Catalyst:
A catalyst will not affect equilibrium position. It will just increase the rate at which equilibrium is reacted and it will increase the rate of the forward and reverse reactions equally.
A catalyst is not used up in the reaction.
Wednesday, May 26, 2010
Factors affecting rate of reaction
The collision theory
The collision theory states that for a chemical reaction to occur the chemicals have to collide, they have to collide with enough energy to react and they have to collide with the correct orientation.
Factors that affect the rate of reaction.
Temperature:
With an increase of temperature the particles have more kinetic energy, this leads more collisions per second.
This means that the rate of reaction increases.
Temperature also changes to amount of particles that have the required activation energy to react. When heated, a greater number of the particles have the required activation energy and so there is a faster reaction rate.
Concentration:
A more concentrated reactant will have more particles in a given volume. This leads to more collisions per second and therefore a faster rate of reaction.
Surface area:
Powdered solid has a greater surface area as there is more surface exposed to collisions. This means that there are more collisions per second so a faster rate of reaction.
Catalyst
A catalyst lowers the activation energy needed for a reaction to take place. This means that a greater number of particles have the required energy needed to react and hence a faster rate of reaction.
A catalyst does not get used up during the reaction.
The collision theory states that for a chemical reaction to occur the chemicals have to collide, they have to collide with enough energy to react and they have to collide with the correct orientation.
Factors that affect the rate of reaction.
Temperature:
With an increase of temperature the particles have more kinetic energy, this leads more collisions per second.
This means that the rate of reaction increases.
Temperature also changes to amount of particles that have the required activation energy to react. When heated, a greater number of the particles have the required activation energy and so there is a faster reaction rate.
Concentration:
A more concentrated reactant will have more particles in a given volume. This leads to more collisions per second and therefore a faster rate of reaction.
Surface area:
Powdered solid has a greater surface area as there is more surface exposed to collisions. This means that there are more collisions per second so a faster rate of reaction.
Catalyst
A catalyst lowers the activation energy needed for a reaction to take place. This means that a greater number of particles have the required energy needed to react and hence a faster rate of reaction.
A catalyst does not get used up during the reaction.
Saturday, May 8, 2010
Internal sample questions and answers
Achieved
1 Calculate the amount (in moles) of carbon dioxide, CO2, in 23.0 g of the gas?
M(CO2) = 44.0 g mol-1
n(CO2) = = 0.523 mol
Achieved
2 Calculate the mass of 2.65 mol of sodium hydroxide, NaOH.
M(NaOH) = 40.0 g mol-1
m = 2.65 mol x 40.0 g mol–1 = 106 g
Achieved
3 24.5 mL of a solution contains 0.00257 mol of HCl. Calculate the concentration, in mol L–1, of the HCl solution.
c = = 0.105 mol L–1
Achieved
4 Calculate the amount (in mole) of NaOH present in 15.6 mL of 0.152 mol L–1 solution.
n = 0.152 mol L–1 x 15.6 x 10–3 L = 2.37 x 10–3 mol
Merit
5(a) Caffeine is a stimulant which has a mass composition of 49.5% carbon, 5.20% hydrogen, 28.9% nitrogen and 16.5% oxygen.
Calculate the empirical formula of caffeine.
(a) n(C) = = 4.13 mol n(H) = = 5.20 mol
n(N) = = 2.06 mol n(O) = = 1.03 mol
empirical formula C4H5N2O
Amount in moles of each element correct.
Correct process, but incorrect empirical formula used. (achieved)
(b) If the molar mass of caffeine is 194 g mol-1, use your answer to part (a) above to determine the molecular formula of caffeine.
molecular formula C8H10N4O2
Empirical and molecular formulae correct. (merit)
Merit
6. Calculate the percentage of carbon and oxygen in sucrose, C12H22O11.
M(C12H22O11) = 342 g mol–1
% C= x 100 = 42.1 %
% O = x 100 = 51.5 %
Process correct but incorrect molar mass of sucrose used. (Achieved)
% of C and O correct(Merit)
Merit
7. 500 mL of 0.253 mol L-1 NaHCO3 solution is mixed with 800 mL of 0.824 mol L-1 NaHCO3 solution.
What is the concentration of the final solution?
n(NaHCO3)
= (0.253 mol L-1 x 0.500 L) + (0.824 mol L-1 x 0.800 L)
= 0.786 mol
Total volume = 1.30 L
concentration of final solution = = 0.604 mol L-1
Correct total amount of NaHCO3
OR
correct process for calculating concentration using a volume of 1.3 L. (Achieved)
Correct determination of concentration of final solution. (Merit)
Excellence
8. A chemist found that 4.69 g of sulfur combined with fluorine t
o produce 15.81 g of gas. Determine the empirical formula of the compound.
n(S) = = 0.146 mol
m(F) = 15.81 - 4.69 = 11.1 g
n(F) = = 0.584 mol
Hence S : F = 1 : 4 and empirical formula is SF4.
Correct amount of S OR F. (achieved)
Correct process with one minor error. eg Incorrect mass of F (Merit)
Correct determination empirical formula. (excellence)
Excellence
9. What mass of CO2 is produced in the complete combustion of 36.5 g of ethanol according to the following equation?
C2H5OH + 3 O2 2 CO2 + 3 H2O
M(C2H5OH) = 46.0 g mol-1
(i) n(C2H5OH) = 36.5 g / 46.0 g mol-1 = 0.793 mol
(ii) n(CO2) = 2 x n(C2H5OH) = 1.59 mol
(iii) m(CO2) = 1.59 mol x 44.0 g mol-1 = 69.8 g
Correct process used in either step (i) or (iii). (achieved)
Correct process with one minor error. eg An incorrect molar mass. (Merit)
Correct answer for mass of CO2 produced. (excellence)
Excellence
10 .What mass of iron can be produced if 50.0 g of carbon monoxide react with iron(III) oxide according to the following equation?
Fe2O3 + 3 CO 2 Fe + 3 CO2
(i) n(CO) = = 1.79 mol
(ii) n(Fe) = x n(CO) = 1.19 mol
(iii) m(Fe) = 1.19 mol x 55.9 g mol-1 = 66.5 g
Correct process used in either step (i) or (iii).(achieved)
Correct process with one minor error.eg An incorrect molar mass.(Merit)
Correct answer for mass of Fe produced. (Excellence)
Excellence
11. Hydrated magnesium sulfate is heated in a crucible. The following data is collected:
mass of crucible and lid = 26.49 g
mass of hydrated magnesium sulfate = 2.13 g
mass crucible, lid and magnesium sulfate after first heating = 27.55 g
mass of crucible, lid and magnesium sulfate after second heating = 27.53 g
Use these results to determine the formula of hydrated magnesium sulfate.
M(MgSO4) = 120.3 g mol-1 M(H2O) = 18.0 g mol-1
mass of anhydrous MgSO4 = 1.04 g
mass of H2O = 1.09 g
amount of MgSO4 = = 8.65 x 10-3 mol
amount H2O = = 6.06 x 10-2 mol
ratio n(H2O) / n(MgSO4) = 7
formula is MgSO4.7H2O
Correct determination of masses of MgSO4 and H2O.(Achieved)
Correct determination of either amount of MgSO4 or H2O. (Merit)
Correct formula for hydrated salt.(Excellence)
1 Calculate the amount (in moles) of carbon dioxide, CO2, in 23.0 g of the gas?
M(CO2) = 44.0 g mol-1
n(CO2) = = 0.523 mol
Achieved
2 Calculate the mass of 2.65 mol of sodium hydroxide, NaOH.
M(NaOH) = 40.0 g mol-1
m = 2.65 mol x 40.0 g mol–1 = 106 g
Achieved
3 24.5 mL of a solution contains 0.00257 mol of HCl. Calculate the concentration, in mol L–1, of the HCl solution.
c = = 0.105 mol L–1
Achieved
4 Calculate the amount (in mole) of NaOH present in 15.6 mL of 0.152 mol L–1 solution.
n = 0.152 mol L–1 x 15.6 x 10–3 L = 2.37 x 10–3 mol
Merit
5(a) Caffeine is a stimulant which has a mass composition of 49.5% carbon, 5.20% hydrogen, 28.9% nitrogen and 16.5% oxygen.
Calculate the empirical formula of caffeine.
(a) n(C) = = 4.13 mol n(H) = = 5.20 mol
n(N) = = 2.06 mol n(O) = = 1.03 mol
empirical formula C4H5N2O
Amount in moles of each element correct.
Correct process, but incorrect empirical formula used. (achieved)
(b) If the molar mass of caffeine is 194 g mol-1, use your answer to part (a) above to determine the molecular formula of caffeine.
molecular formula C8H10N4O2
Empirical and molecular formulae correct. (merit)
Merit
6. Calculate the percentage of carbon and oxygen in sucrose, C12H22O11.
M(C12H22O11) = 342 g mol–1
% C= x 100 = 42.1 %
% O = x 100 = 51.5 %
Process correct but incorrect molar mass of sucrose used. (Achieved)
% of C and O correct(Merit)
Merit
7. 500 mL of 0.253 mol L-1 NaHCO3 solution is mixed with 800 mL of 0.824 mol L-1 NaHCO3 solution.
What is the concentration of the final solution?
n(NaHCO3)
= (0.253 mol L-1 x 0.500 L) + (0.824 mol L-1 x 0.800 L)
= 0.786 mol
Total volume = 1.30 L
concentration of final solution = = 0.604 mol L-1
Correct total amount of NaHCO3
OR
correct process for calculating concentration using a volume of 1.3 L. (Achieved)
Correct determination of concentration of final solution. (Merit)
Excellence
8. A chemist found that 4.69 g of sulfur combined with fluorine t
o produce 15.81 g of gas. Determine the empirical formula of the compound.
n(S) = = 0.146 mol
m(F) = 15.81 - 4.69 = 11.1 g
n(F) = = 0.584 mol
Hence S : F = 1 : 4 and empirical formula is SF4.
Correct amount of S OR F. (achieved)
Correct process with one minor error. eg Incorrect mass of F (Merit)
Correct determination empirical formula. (excellence)
Excellence
9. What mass of CO2 is produced in the complete combustion of 36.5 g of ethanol according to the following equation?
C2H5OH + 3 O2 2 CO2 + 3 H2O
M(C2H5OH) = 46.0 g mol-1
(i) n(C2H5OH) = 36.5 g / 46.0 g mol-1 = 0.793 mol
(ii) n(CO2) = 2 x n(C2H5OH) = 1.59 mol
(iii) m(CO2) = 1.59 mol x 44.0 g mol-1 = 69.8 g
Correct process used in either step (i) or (iii). (achieved)
Correct process with one minor error. eg An incorrect molar mass. (Merit)
Correct answer for mass of CO2 produced. (excellence)
Excellence
10 .What mass of iron can be produced if 50.0 g of carbon monoxide react with iron(III) oxide according to the following equation?
Fe2O3 + 3 CO 2 Fe + 3 CO2
(i) n(CO) = = 1.79 mol
(ii) n(Fe) = x n(CO) = 1.19 mol
(iii) m(Fe) = 1.19 mol x 55.9 g mol-1 = 66.5 g
Correct process used in either step (i) or (iii).(achieved)
Correct process with one minor error.eg An incorrect molar mass.(Merit)
Correct answer for mass of Fe produced. (Excellence)
Excellence
11. Hydrated magnesium sulfate is heated in a crucible. The following data is collected:
mass of crucible and lid = 26.49 g
mass of hydrated magnesium sulfate = 2.13 g
mass crucible, lid and magnesium sulfate after first heating = 27.55 g
mass of crucible, lid and magnesium sulfate after second heating = 27.53 g
Use these results to determine the formula of hydrated magnesium sulfate.
M(MgSO4) = 120.3 g mol-1 M(H2O) = 18.0 g mol-1
mass of anhydrous MgSO4 = 1.04 g
mass of H2O = 1.09 g
amount of MgSO4 = = 8.65 x 10-3 mol
amount H2O = = 6.06 x 10-2 mol
ratio n(H2O) / n(MgSO4) = 7
formula is MgSO4.7H2O
Correct determination of masses of MgSO4 and H2O.(Achieved)
Correct determination of either amount of MgSO4 or H2O. (Merit)
Correct formula for hydrated salt.(Excellence)
Monday, March 29, 2010
Types of particles in given substances
Summary:
Metallic substances contain atoms
Ionic substances contain ions
Giant covalent substances contain atoms
Simple covalent molecules contain atoms
Solid /Type of particle / Attractive force between particles
sodium oxide / ions / intramolecular ionic bond
sulfur trioxide / molecules / intermolecule dipole-dipole interaction
silicon dioxide / atoms / intramolecular covalent bonds
aluminium oxide / ions / intramolecular ionic bonds
Metallic substances contain atoms
Ionic substances contain ions
Giant covalent substances contain atoms
Simple covalent molecules contain atoms
Solid /Type of particle / Attractive force between particles
sodium oxide / ions / intramolecular ionic bond
sulfur trioxide / molecules / intermolecule dipole-dipole interaction
silicon dioxide / atoms / intramolecular covalent bonds
aluminium oxide / ions / intramolecular ionic bonds
Bond polarity questions
To work out bond polarity you need to do the following:
1. Draw the lewis diagram.
2. Work out the shape.
3. check that the bond polarities cancel out. Is the a net dipole moment?
in your answer for explaining polarity... include the following words.
polar bonds, electronegativity, net dipole, symmetrical, asymmetrical
Remember:
symmetrical molecules = non-polar
asymmetrical molecules = polar
1. Draw the lewis diagram.
2. Work out the shape.
3. check that the bond polarities cancel out. Is the a net dipole moment?
in your answer for explaining polarity... include the following words.
polar bonds, electronegativity, net dipole, symmetrical, asymmetrical
Remember:
symmetrical molecules = non-polar
asymmetrical molecules = polar
Test and Exam hints for Bonding
When you are answering questions about the properties of materials you need to do the following:
1. work out the type of substance.
2. write an intro paragraph that describes the structure and bonding in the structure. (below)
3. Link the structure to the property in the question.
Starter paragraphs for each type of substances are as follows.
Learn these
Metallic substances.
Metals are made up of metal atoms stacked into a 3 dimensional lattice held together by strong electrostatic attraction betweent the atom and its valence electrons This attraction is nondirectional. These valence electrons form a sea of electrons.
Ionic structures
Ionic crystals are made up of a 3 dimensional lattice of alternating positive cations and negative ions held together by strong diredtional electrostatic attraction between the ions.
Giant covalent structures
(Diamond, Graphite, Buck balls and silicon dioxide only)
These structures are made up of atoms covalently bonded together in a 3 dimensional structure.
Diamond is made up of carbon atoms bonded to 4 other C atoms.
Graphite is made up of carbon atoms bonded in layers to 3 other C atoms, leaving a free electron wh ich is found between the graphite layers.
Simple covalent molecules
These are made up of strong intramolecular covelant bonds and weak intermolecular Van der Waals forces. These Van Der Waal forces are weak and very easy to break so not much energy is needed to overcome these forces.
Van Der Waals forces:
Dipole-dipole interactions
Instantaneous dipole interactions
H bonding (molecules that have H with O, N, F atoms)
1. work out the type of substance.
2. write an intro paragraph that describes the structure and bonding in the structure. (below)
3. Link the structure to the property in the question.
Starter paragraphs for each type of substances are as follows.
Learn these
Metallic substances.
Metals are made up of metal atoms stacked into a 3 dimensional lattice held together by strong electrostatic attraction betweent the atom and its valence electrons This attraction is nondirectional. These valence electrons form a sea of electrons.
Ionic structures
Ionic crystals are made up of a 3 dimensional lattice of alternating positive cations and negative ions held together by strong diredtional electrostatic attraction between the ions.
Giant covalent structures
(Diamond, Graphite, Buck balls and silicon dioxide only)
These structures are made up of atoms covalently bonded together in a 3 dimensional structure.
Diamond is made up of carbon atoms bonded to 4 other C atoms.
Graphite is made up of carbon atoms bonded in layers to 3 other C atoms, leaving a free electron wh ich is found between the graphite layers.
Simple covalent molecules
These are made up of strong intramolecular covelant bonds and weak intermolecular Van der Waals forces. These Van Der Waal forces are weak and very easy to break so not much energy is needed to overcome these forces.
Van Der Waals forces:
Dipole-dipole interactions
Instantaneous dipole interactions
H bonding (molecules that have H with O, N, F atoms)
Tuesday, March 23, 2010
Wiki questions
http://tll-chem-science.wikispaces.com
Metals structure and its properties
Copper can be beaten out into thin sheets without shattering into pieces.
(a) Name this property.
(b) Explain how this property is related to the metallic structure and bonding of Copper.
Simple covalent molecules and shapes of molecules
The molecules CH2O and NH3 both consist of a central atom with three other atoms bonded to it covalently. In spite of this similarity, the shapes and bond angles of the two molecules are quite different.
a) Discuss the reasons for this.
b) Explain why BH3 is trigonal planar while CH4 is tetrahedral.
Properties of Materials
Discuss with reference to structure and bonding:
a) Chlorine is a gas at room temperature but sodium chloride is solid
b) Ammonia has a bond angle of 107º whereas the bond angles in water are only 105º.
Metals structure and its properties
Copper can be beaten out into thin sheets without shattering into pieces.
(a) Name this property.
(b) Explain how this property is related to the metallic structure and bonding of Copper.
Simple covalent molecules and shapes of molecules
The molecules CH2O and NH3 both consist of a central atom with three other atoms bonded to it covalently. In spite of this similarity, the shapes and bond angles of the two molecules are quite different.
a) Discuss the reasons for this.
b) Explain why BH3 is trigonal planar while CH4 is tetrahedral.
Properties of Materials
Discuss with reference to structure and bonding:
a) Chlorine is a gas at room temperature but sodium chloride is solid
b) Ammonia has a bond angle of 107º whereas the bond angles in water are only 105º.
Saturday, March 20, 2010
covalent bonding
This is an animation of two hydrogen atoms forming a covalent bond by sharing it valence electrons in the formation of a covalent bond.
Tuesday, March 9, 2010
Tuesday, March 2, 2010
Sunday, February 28, 2010
Isotopes
Saturday, February 27, 2010
Atomic structure
Tuesday, February 16, 2010
Odd bits and pieces.
1. Reactions in your internal that do not follow the rules.
When you react silver ions (Ag+) with sodium hydroxide (NaOH) a brown precipitate of silver oxide is formed instead of the expected silver hydroxide.
Ag+(aq) + OH-(aq) --> Ag2O(s).
2.Other odd bits:
When you are identifying chloride ions in a solution of copper chloride CuCl2.
The addition of silver nitrate to identify the Cl- ions forms a white ppt of silver chloride.
Ag+(aq) + Cl-(aq) --> AgCl(s)
When ammonia is added to this white ppt of silver chloride the ppt will disappear, BUT
The addition of ammonia will also result in the formation of the following ppt:
Cu2+ + OH- --> Cu(OH)2 (light blue ppt)
So the ppt of silver chloride disappears and the silver complex ion [Ag(NH3)2]+(aq) is formed. But a light blue ppt of Cu(OH)2(s) remains.
You will observe a white ppt turning from white AgCl to light blue Cu(OH)2.
3. Left out bits from your notes:
When your add potassium thiocynate to iron (III) ions, a complex ion is formed that is blood red in colour.
Fe3+(aq) + SCN-(aq) --> [Fe(SCN)]2+ (aq).
4. A confirming test for I-(aq) ions
If you are unsure if you have Cl- or I- ions, then you can check by adding lead ions (Pb2+)
If you have I- ions then you will get a bright yellow ppt of PbI2(S)
When you react silver ions (Ag+) with sodium hydroxide (NaOH) a brown precipitate of silver oxide is formed instead of the expected silver hydroxide.
Ag+(aq) + OH-(aq) --> Ag2O(s).
2.Other odd bits:
When you are identifying chloride ions in a solution of copper chloride CuCl2.
The addition of silver nitrate to identify the Cl- ions forms a white ppt of silver chloride.
Ag+(aq) + Cl-(aq) --> AgCl(s)
When ammonia is added to this white ppt of silver chloride the ppt will disappear, BUT
The addition of ammonia will also result in the formation of the following ppt:
Cu2+ + OH- --> Cu(OH)2 (light blue ppt)
So the ppt of silver chloride disappears and the silver complex ion [Ag(NH3)2]+(aq) is formed. But a light blue ppt of Cu(OH)2(s) remains.
You will observe a white ppt turning from white AgCl to light blue Cu(OH)2.
3. Left out bits from your notes:
When your add potassium thiocynate to iron (III) ions, a complex ion is formed that is blood red in colour.
Fe3+(aq) + SCN-(aq) --> [Fe(SCN)]2+ (aq).
4. A confirming test for I-(aq) ions
If you are unsure if you have Cl- or I- ions, then you can check by adding lead ions (Pb2+)
If you have I- ions then you will get a bright yellow ppt of PbI2(S)
Wednesday, February 10, 2010
Hints for your internal
1. Write down EVERY test you do on each unknown
2. Write an ionic equation foe EVERY precipitate that you form and EVERY complex ion that you form.
3. If you are not sure about the state symbols [(s), (aq)] then leave them out of your equations.
2. Write an ionic equation foe EVERY precipitate that you form and EVERY complex ion that you form.
3. If you are not sure about the state symbols [(s), (aq)] then leave them out of your equations.
Wednesday, February 3, 2010
Welcome
Subscribe to:
Posts (Atom)