The titration sequence of events:
1. Rinse a conical flask with water.
2. Rinse the pipette with the HCl solution and then pipette, a 25 mL sample of the hydrochloric acid and place it in a conical flask, (an aliquot). Repeat so that you have 3 conical flasks set up and ready to go.
A pipette is a piece of apparatus which accurately delivers a given volume -before filling it is always rinsed with a small amount of the solution.
3. A few drops of an appropriate acid-base indicator, in this case phenolphthalein, is added to the flask. The solution will remain colourless.
4. A burette is then rinsed with a sample of sodium hydroxide (the solution it is to be filled with) and then filled to just below the 0.00 mL mark
A burette is another piece of glassware that accurately measures out a volume of liquid.
5. The initial volume of aqueous NaOH in the burette is carefully read (to 2 decimal places - giving an accuracy of ± 0.02 mL).
6. Carefully add the NaOH to the aqueous HCl, finishing the titration as soon as the first permanent pink colour is observed.
The colour change is referred to as the end-point, and assuming the correct indicator has been used, is the point when the acid and base have reacted in the molar ratio given by the balanced neutralisation equation.
7. By taking the difference between the initial and final volumes in the burette, the total volume of NaOH(aq) added can be carefully determined.
8. The titration is repeated at least 3 times, or until you have obtained 3 concordant results i.e. burette volumes (titres) that, ideally, agree to within ± 0.2 mL.
Monday, June 28, 2010
Calculations
Rules and regulations:
You must record your calculations to as many sif figs as are on the calculator.
These sig figs must then be round down to 3 sig fig for your final answer.
You must record your calculations to as many sif figs as are on the calculator.
These sig figs must then be round down to 3 sig fig for your final answer.
Titrations
What is a titration?
In an acid-base titration the neutralisation reaction between an acid and base is used to determine the concentration of one of the reactants, if the concentration of the other is accurately known.
Using the reaction between a solution of hydrochloric acid (unknown concentration) and sodium hydroxide (standard solution - concentration known) is an example.

For achievement with excellence:
• at least three titre values must fall within a range of 0.2 mL; the average titre value must be within 0.2 mL of the expected outcome
• a titration calculation where the stoichiometry is not one-to-one must be carried out correctly using only concordant titre values. The final answer must have correct units and an appropriate number of significant figures.
In an acid-base titration the neutralisation reaction between an acid and base is used to determine the concentration of one of the reactants, if the concentration of the other is accurately known.
Using the reaction between a solution of hydrochloric acid (unknown concentration) and sodium hydroxide (standard solution - concentration known) is an example.

For achievement with excellence:
• at least three titre values must fall within a range of 0.2 mL; the average titre value must be within 0.2 mL of the expected outcome
• a titration calculation where the stoichiometry is not one-to-one must be carried out correctly using only concordant titre values. The final answer must have correct units and an appropriate number of significant figures.
Tuesday, June 15, 2010
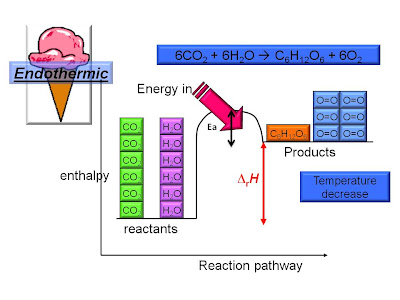
Measuring Reaction Enthalpy
Equilibrium
Kc Equilibrium constant:
eg for: N2 + 3H2 --> 2NH3
Kc = [product]/[reactant]
Kc = [NH3]2/[N2][H2]3
This is a ratio of product concentration to reactant concentration at equilibrium.
If Kc>1 then the equuilibrium position favours the product side
If Kc<1 then the equilibrium position favours the reactant side.
* Solids do NOT get included into the Kc expression
Le Chateliers Principle:
States that:
When a system is at equilibrium, that system will shift to minimise any changes made to it.
[When answering questions on equilibrium, you need to name the chemical(s) affected by the change]
Things that can change the position of equilibrium:
1. Temperature:
When heat is added, the equilibrium will shift in the endothermic direction.
When cooled the equilibrium will shift in the exothermic direction.
Kc will change as it is temperature dependant.
2. Concentration:
When adding or removing chemicals from an equilibrium you are changing the concentration. The equilibrium will shift in the direction to either replace any chemicals removed or use up any chemical added.
Kc remains the same.
3. Pressure:
When the pressure is increased (by decreasing the volume of the container) the equilibrium will shift to the side with the least number of moles of gas.
When the pressure is decreased (by increasing the volume of the container) the equilibrium will shift to the side with the most moles of gas.
Kc remains the same.
4. Catalyst:
A catalyst will not affect equilibrium position. It will just increase the rate at which equilibrium is reacted and it will increase the rate of the forward and reverse reactions equally.
A catalyst is not used up in the reaction.
eg for: N2 + 3H2 --> 2NH3
Kc = [product]/[reactant]
Kc = [NH3]2/[N2][H2]3
This is a ratio of product concentration to reactant concentration at equilibrium.
If Kc>1 then the equuilibrium position favours the product side
If Kc<1 then the equilibrium position favours the reactant side.
* Solids do NOT get included into the Kc expression
Le Chateliers Principle:
States that:
When a system is at equilibrium, that system will shift to minimise any changes made to it.
[When answering questions on equilibrium, you need to name the chemical(s) affected by the change]
Things that can change the position of equilibrium:
1. Temperature:
When heat is added, the equilibrium will shift in the endothermic direction.
When cooled the equilibrium will shift in the exothermic direction.
Kc will change as it is temperature dependant.
2. Concentration:
When adding or removing chemicals from an equilibrium you are changing the concentration. The equilibrium will shift in the direction to either replace any chemicals removed or use up any chemical added.
Kc remains the same.
3. Pressure:
When the pressure is increased (by decreasing the volume of the container) the equilibrium will shift to the side with the least number of moles of gas.
When the pressure is decreased (by increasing the volume of the container) the equilibrium will shift to the side with the most moles of gas.
Kc remains the same.
4. Catalyst:
A catalyst will not affect equilibrium position. It will just increase the rate at which equilibrium is reacted and it will increase the rate of the forward and reverse reactions equally.
A catalyst is not used up in the reaction.
Subscribe to:
Comments (Atom)